Medical alcohol is completely identical to technical alcohol in such qualities as smell and color. However, there is an important difference between them. The technical composition contains methyl, a substance that can cause severe poisoning and cause death. Unlike technical alcohol, the main component in medical alcohol is ethyl, which is also a poison, but still its use in moderate doses does not cause such terrible consequences. In this article we will tell you in detail what ethyl alcohol and medical alcohol are.
Medical alcohol is one of the few subtypes of ethanol that has a monoatomic structure. The composition of medical ethyl alcohol consists of four percent water and ninety-six percent alcohol.
Thanks to this composition, medical alcohol has become extremely popular. It is used not only for medical purposes, but also for industrial purposes. Very often it is used internally, but this requires diluting it. Ethanol alcohol has the form of a clear liquid and is sold in any pharmacy. The dosage can be from one hundred milligrams or more.
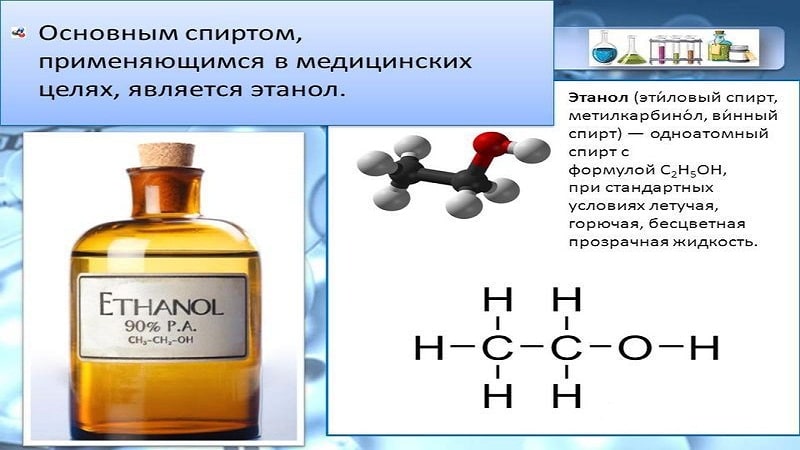
Ethanol under standard conditions is a volatile, flammable, colorless, transparent liquid.
Only food raw materials are used for its production. Typically these products are:
- potato;
- barley;
- oats;
- corn.
Very often, specialists are forced to answer the question: medical alcohol and ethyl alcohol, is there a difference? To the average person, the difference between these two compositions is not noticeable. Both compositions have the same formula, but are made from different natural ingredients. The ethyl compound is also used in alcohol. So, to create wine, a composition based on grapes or berries is used.
The technical type of alcohol is produced using a special technology, when the active substance undergoes a decomposition process as a result of treatment with water. Some types of wood and petroleum products can act as the active substance. In most cases, the resulting type of alcohol is used as a fuel or solvent.
Wine, ethyl, medical - compositions in which the main active substance is ethyl. Despite the fact that all these types have the same structure, they undergo different degrees of purification. Medical alcohol is a solution that has the highest degree of purification, and this is why it is so widely used. It can easily be diluted with substances such as:
- water;
- glycerol;
- acetic acid.

Ethyl alcohol is used as a fuel, as a solvent, as a filler in alcohol thermometers, and as a disinfectant.
Application
In most cases, such a solution is used in medicine and is used for disinfection. However, very often homemade alcohol is produced using this base.
In medicine, alcohol solutions are used as:
- Antiseptic. For treating scratches, cuts and other wounds.
- A substance that has disinfectant properties. Treatment with this composition destroys up to ninety-seven percent of all existing bacteria and infections on the skin.
- Anesthesia. Under field conditions of surgical intervention.
- Main component used when creating tinctures.
- Rubbing alcohol is often used when creating compresses and antipyretics.
- The drug is used as one of the main components mechanical ventilation procedures(Artificial Lung Ventilation).
Using alcohol as a disinfectant, it is used to treat skin lesions, surgical instruments, and even surgical fields. To do this, a cotton swab is generously moistened with liquid and applied to the desired area.
In case of poisoning, industrial alcohol can be a fairly effective antidote. Of all the types based on ethanol, only medical alcohol is suitable for these purposes. Timely consumption orally can reduce the concentration of toxins in the body.

There are 2 main ways to produce ethanol - microbiological (alcoholic fermentation) and synthetic (ethylene hydration
Alcohol, which has an ethyl base, is one of the essential substances in medicine. Every medical procedure involves its use. However, to achieve different goals, different strengths of the substance are used; it can be forty, seventy and ninety percent.
Ethyl alcohol is a versatile product used in many industrial sectors. Alcoholic drinks, kvass, kefir and even non-alcoholic beer are created on its basis. However, in fermented milk products its concentration does not exceed one tenth of a percent. That is why consuming such products does not harm the body. Very often the solution is used as a preservative in the manufacture of confectionery and bakery products.
Rubbing alcohol is often consumed by people suffering from alcohol addiction. Since purchasing the product does not require a doctor’s prescription, it has become widespread among people with this addiction. Drinking medical alcohol in its pure form can cause a burn to the throat and gastric mucosa. Ingesting medical alcohol must be diluted, and its strength should not exceed fifty degrees. Even taking into account the fact that medical alcohol contains only plant components, its excessive consumption leads to the development of serious diseases.
Harm
Few people know, but alcohol sold in pharmacies has specific instructions for use. These instructions indicate that the main function of the composition is disinfection of the skin. Experts categorically prohibit the use of ethanol for treating skin exposed to inflammation. The warming effect can play a negative role and these processes will worsen.
The productivity of a modern distillery is about 30,000-100,000 liters of alcohol per day
The development of an allergic reaction of the body is possible, therefore the product is not recommended for use by persons under fourteen years of age. For women during pregnancy or breastfeeding, it is best to avoid using medical alcohol. As a result of weakened immunity, applying the solution to the skin may cause irritation. If the area of skin treated with alcohol turns red after the procedure, it must be rinsed with clean water. If such reactions occur in the body, you should stop using the drug.
Applying alcohol to delicate areas of the skin, such as the eyelids, can cause not only a burn to the skin, but also to the mucous membrane of the eyeball. In cases where the composition is not used for its intended purpose, consequences such as poisoning with toxins and even a narcotic effect are possible. In most cases, these reactions have a direct relationship with the amount and method of application of the composition.
An overdose caused by consuming or inhaling ethanol in huge concentrations can cause disruption in the functioning of the nervous system. Such consequences can lead to severe intoxication, emotional stupor and even coma. It is important to seek medical help when the first symptoms of toxin poisoning appear.
Excessive consumption of alcoholic beverages is addictive. When drinking alcohol, the human body produces the hormone endorphin, which is the main reason for the development of alcoholism. It is important to remember that ethanol is a toxic substance. Its single dose should not exceed three grams per kilogram of live weight. Exceeding this dose can cause poisoning and lead to coma. Abuse of alcoholic beverages leads to the development of serious diseases of the liver and stomach. So, as a result of the effect of alcohol on the body, diseases such as:
- stomach ulcer;
- gastritis;
- cirrhosis;
- cancer of internal organs.
Very often, excessive consumption of medical alcohol causes the development of cardiovascular disorders.

On an industrial scale, ethyl alcohol is produced from raw materials containing cellulose (wood, straw), which is previously hydrolyzed
Alcohol addiction causes pathologies in the functioning of parts of the brain. Its influence has a destructive effect on the condition of cells and neurons. As a result of prolonged internal use of medical alcohol, mental disorders may begin to develop.
Changes occurring in the body have a strong impact on the state of the nervous system. With such disorders, depression, apathy and suicidal tendencies may occur. Medical alcohol must be used only for its intended purpose, observing the expiration date of the product.


